ePostcard #133: Glass in Nature (Part 8)
Photo Credit: Popigai diamonds courtesy of The Siberian Times (siberiantimes.com).
DIAMONDS IN THE ROUGH
Throughout its history, Earth has been repeatedly hit by large meteorites. Of the more than 60,000 meteorites that have been discovered on Earth, most come from the Asteroid Belt. If a meteorite strike is large enough to form an “astrobleme” (from Greek astron ‘star’ + blēma ‘wound’), the bedrock, sand, or soil beneath and surrounding the impact site typically undergoes dramatic deformation (or meteoric metamorphism) as a result of the tremendous heat, pressure and shock waves associated with the strike. The high temperatures and pressure conditions of the impact are similar to those that create diamonds deep in the Earth’s interior, especially if the “target” rock material in the impact zone contains carbon.
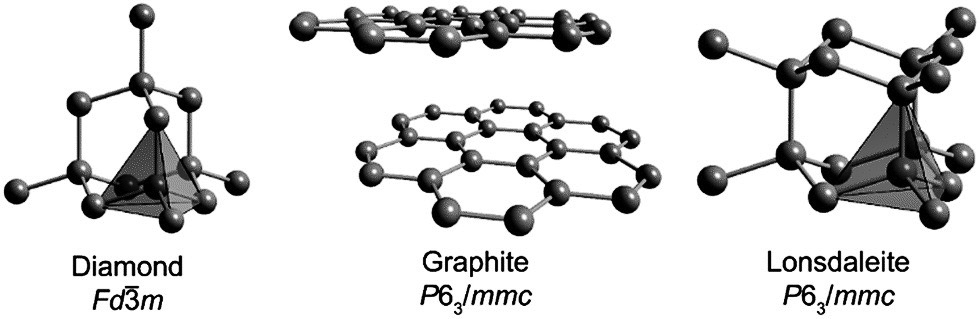
Diamonds have been found in and around the craters of many impact sites. Geologists had long suspected that if a meteorite smashed into Earth hard enough, it could transform graphite, a type of soft, pure carbon, into a mineral even harder than a diamond. How can one mineral become another? Both graphite and diamond are made of carbon atoms bound to each other. In the soft, gray graphite (the very same mineral that is in your pencil), the carbon atoms are arranged in sheets. In deep-earth diamonds, on the other hand, the atoms are arranged in repeating cubes. As you can see in the diagram below, the arrangement of the carbon atoms results in a different crystal structure.
Illustration Credit: Crystal structures of diamond, graphite and lonsdaleite courtesy of Popov I.V., Gorne A.L., Tchougreeff A.L., Dronskowski R. (2019): Relative stability of diamond and graphite as seen through bonds and hybridizations, Physical Chemistry and Chemical Physics., 21, 10961-10969. 2019; Gulf Institute of Gemology.
How are meteorite diamonds different from the gem-quality diamonds we are most familiar with? Diamonds are a solid form of carbon with a distinctive cubic crystal structure. They are generally formed at depths of 100 to 150 miles in the Earth’s mantle, although a few have come from as deep as 500 to 600 miles down. These super-deep Earth diamonds form in a fiery cauldron of carbon-rich magma at up to 1,000 degrees F and at 240,000 times the atmospheric pressure at sea level. Many geologists believe that most of the “diamonds in the rough” mined commercially formed over 3.5 billion years ago in the Earth’s mantle, where conditions of extreme heat and pressure caused the carbon atoms in the primordial magma to crystallize into the distinctive octahedrons and cubes typical of diamonds. The carbon source for these deep-earth diamonds was most likely carbon trapped in Earth’s interior at the time of our planet’s formation. The dense crystalline structure, in which each carbon atom is linked to 4 equidistant neighbors, is what gives these “mantle diamonds” their unsurpassed hardness and many other properties.
How did these ancient diamonds reach the Earth’s surface? The answer is as amazing as the crystal perfection of the diamonds themselves. It is believed that magma generation (the melting of the mineral peridotite) in the upper layers of the mantle due to thermal convection and the triggering mechanism of plate tectonics resulted in violent, deeply-sourced volcanic eruptions. These explosive eruptions created the “magmatic plumbing” systems that provided the pathways for the diamondiferous magmas (kimberlites) to reach the surface. These volcanic eruptions were far more explosive, breaching the Earth’s crust with much higher velocities than the volcanic activity we see today in places like Hawaii, Iceland, Indonesia, and Mount St. Helens. What is so fascinating to me is that these diamonds were essentially “pre-crystallized hitchhikers” in the volcanic kimberlite magmas that brought them to the surface, their crystalline perfection unmarred by their fiery ascent.
Photo Credit: Courtesy of Wikimedia Creative Commons License. This photo of Kimberlite, the volcanic rock that is found in many diamondiferous pipes, is from the Finsch Diamond Mine in South Africa. This specimen includes several tiny diamonds and a lovely octahedral diamond crystal of about 1.8 carats. Kimberlite is a variety of peridotite, and much of the Earth’s mantle is believed to be composed of peridotite. Peridotites are economically important rocks because they are the source for many of the world’s mined diamonds and often contain chromite – the only ore of chromium. They are also currently being studied by climate change researchers as a means of sequestering carbon dioxide.
EXTRATERRESTRIAL DIAMONDS
About 35 million years ago (and possibly linked to the Eocene–Oligocene mass extinction event), an asteroid about 4 to 5 miles in diameter and traveling at a speed of about 15 to 20 miles per second, slammed into the area that is now known as the Taymyr Peninsula of northern Siberia, Russia. The Popigai Crater (or “Popigai Astroblem”) is the seventh-largest impact crater on Earth. The energy delivered by this hypervelocity impact instantly melted thousands of cubic miles of rock and blasted millions of tons of ejecta high into the air. Some of that ejecta landed on other continents. The explosion created a 62 mile-wide impact crater with an expansive rim of deformed rock more than 12 miles wide. Within this zone, flakes of graphite in the Archean graphite-garnet gneiss were instantly converted into diamond. Researchers estimate that this shell of diamond-bearing rock had a volume of about 1600 cubic miles and contained more diamonds than all of the Earth’s other known deposits combined. Polycrystalline industrial-grade diamonds up to half an inch in size have been found at the Popigai Crater and the crater impact zone is designated by UNESCO as a Geopark, a site of special geological significance because of its motherlode of associated diamonds.
POPIGAI CRATER (RUSSIAN SIBERIA)
Photo Credit: Landsat photo of Popigai Crater in Siberia courtesy of NASA and the Soviet/Russian Space Agency. The Popigai Crater, a source of meteoritic diamonds and impact breccia, is situated at the northern edge of Siberia, on the Tamyr Peninsula, far north of the Arctic Circle.
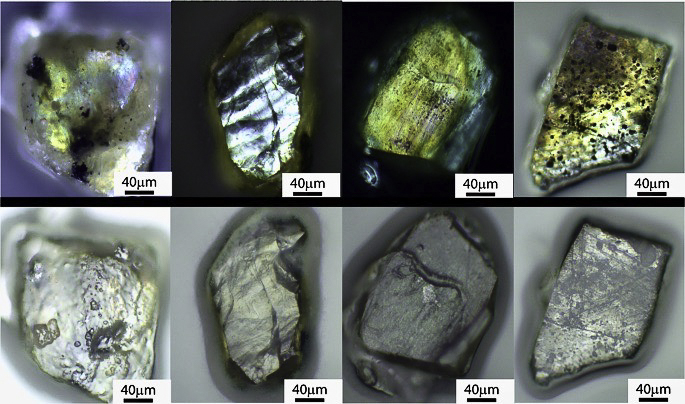
Photo Credit: Courtesy of Murri, M., Smith, R.L., McColl, K. et al. Quantifying hexagonal stacking in diamond. Sci Rep 9, 10334 (2019) and Creative Commons Attribution 4.0 International License. Optical images of representative Popigai impact diamonds examined in this study obtained using (top) transmitted and (bottom) reflected light. Striations in the images correspond to the sheeting of the carbon atoms and the black spots are residual graphite.
ARIZONA’S METEOR (BARRINGER) CRATER
Photo Credits: The two photos of the Barringer Crater above are courtesy of the International Space Station Image Collection; eol.jsc.nasa.gov.
Photo Credit: Courtesy of arizonaattractions.com. Arizona’s Meteor Crater (also known as Barringer Crater) is the best-preserved meteorite impact site on Earth. This spectacular crater is well worth a visit, and is located near Winslow, Arizona, just minutes from Interstate 40 and the old Route 66.
Arizona’s Meteor Crater, also known as the Barringer Crater, is the best-preserved meteorite impact site on Earth. The crater was named in recognition of Daniel Moreau Barringer, the geologist who established its impact origin. We now know that the Canyon Diablo meteorite that created the crater was traveling faster than 22,000 miles per hour when it smashed into the Colorado Plateau (in northern Arizona) nearly 50,000 years ago. The damage inflicted by the nearly 164-foot diameter iron asteroid was similar to a nuclear bomb blast, but without ionizing radiation damage. The massive explosion excavated 175 million tons of rock, forming a crater nearly a mile wide and 570 feet deep. While the impact event was too small to cause global environmental effects, its regional damage would have been significant. Paleoenvironmental data suggests that a juniper-pinyon woodland or forest covered the gently rolling countryside at the time the crater formed. Large mammals such as mammoths, large ground sloths, bison, camels, tapirs and horses may have lived in the vicinity and been victims of the 20 to 40 megaton blast. The asteroid, bedrock, and any fauna or flora at ground zero would have been vaporized.
LONSDALEITE DIAMONDS
About 50 years ago, scientists discovered a new hexagonal form of diamond in the iron-rich fragments of the Canyon Diablo meteorite. They named it lonsdaleite, after Dame Kathleen Lonsdale, a famous British crystallographer. Lonsdaleite has the regular cubic form of a diamond, but exhibits defects within its crystal structure that suggest that it was subjected to extreme shock or pressure during its creation. Researchers have attributed the diamond’s formation to shock-induced transformation of graphite within the meteorite upon impact with the Earth. The meteorite’s violent impact generates incredible heat and pressure, transforming the graphite into a diamond while retaining the graphite’s original hexagonal structure. Since then, “lonsdaleite” has been widely used by scientists as an indicator of ancient asteroidal impacts on Earth, including those linked to mass extinctions. In addition, Lonsdaleite is also thought to have mechanical properties superior to ordinary diamond, giving it high potential industrial significance.
Photo Credit: Kathleen Lonsdale with crystal models, photographer unknown, c. 1946. Courtesy of Prof. Ian Wood, UCL Earth Sciences, for “Disrupters and Innovators: Journeys in gender equality at UCL”, Exhibition 3 at the Octagon Gallery (2019). Kathleen Lonsdale was the crystallographer who established the structure of benzene by X-ray diffraction methods in 1929. She also worked on the synthesis of diamonds, and was a pioneer in the use of X-rays to study crystals.
Photo Credit: Courtesy of Laurence Garvie/Arizona State University (School of Earth and Space Exploration at ASU). These microscopic Lonsdaleite diamonds were found at the Barringer Crater. The atomic-scale structure of normal diamonds is cubic. Lonsdaleite, in contrast, has a hexagonal structure. In addition to the Barringer/Meteor Crater in Arizona, Lonsdaleite diamonds have been associated with the Popigai Crater (Russia), the Tunguska Event, and several other impact crater sites.
Among many research efforts to understand how Lonsdaleite forms, one of the most important was published in Nature Communications by Péter Németh and co-authors Laurence Garvie, Toshihiro Aoki, Peter Buseck, Natalia Dubrovinskaia and Leonid Dubrovinsky. According to lead author Péter Németh, “The so-called lonsdaleite diamond is actually the long-familiar cubic form of diamond, but it’s full of defects. These can occur due to shock metamorphism, plastic deformation or unequilibrated crystal growth.” Using the advanced electron microscopes in ASU’s Center for Solid State Science, the team discovered, both in the Canyon Diablo and the synthetic samples made in the laboratory, new types of diamond twins with features attributed to lonsdaleite. “Most crystals have regular repeating structures, much like the bricks in a well-built wall,” says co-author Peter Buseck. However, interruptions can occur in the regularity, and these are called defects. Defects are intermixed with the normal diamond structure, just as if the wall had an occasional half-brick or longer brick or row of bricks that’s slightly displaced to one side or another.”
In another landmark study, focused on the shock-induced transition from graphite to diamond, researchers at Stanford University’s National Accelerator Laboratory, subjected pyrolytic graphite to a combination of high-energy lasers and ultrafast X-ray diffraction in order to obtain structure measurements delineating the shock-induced compression of graphite to diamond. Their results showed nanosecond formation of the diamond structure after shock compression starting at a high pressure threshold of 50 GPa and the direct formation of lonsdaleite diamonds pressures above 170 GPa,
Source: Kraus, D. et al. Nanosecond formation of diamond and lonsdaleite by shock compression of graphite. Nature Communications. 7:10970 doi: 10.1038/ncomms10970 (2016).
NANODIAMONDS
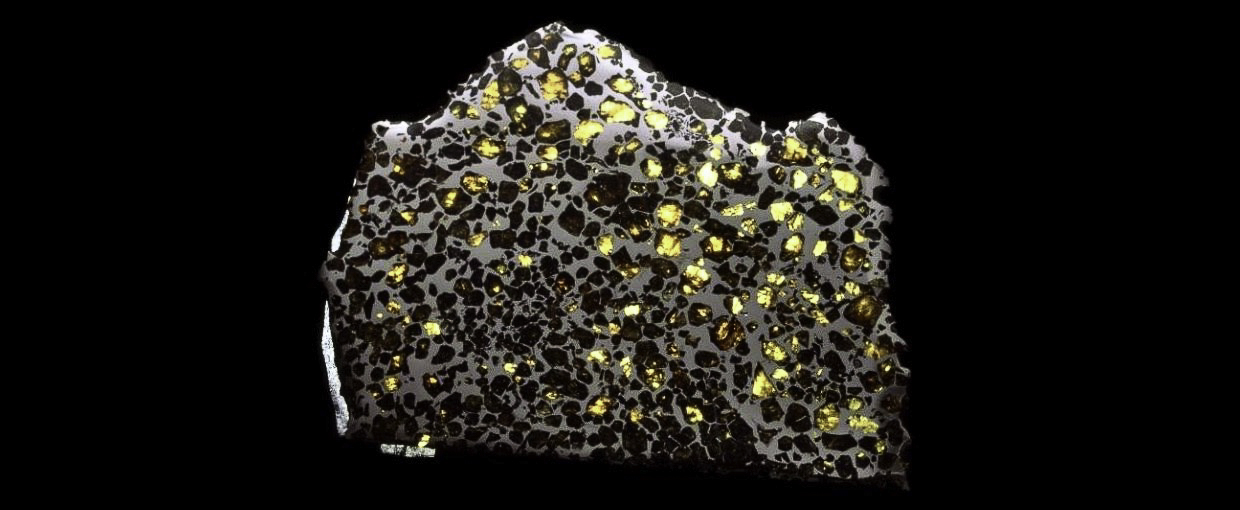
Photo Credit: Courtesy of NASA and the Trustees of the Natural History Museum in London. This photo shows a polished, enlarged section of the Esquel pallasite meteorite that delivered tiny nano-diamonds to Earth. This is a common occurrence, as there is believed to be substantial amounts of high-pressure carbon in the galaxies, and thus some diamonds.
In the 1980s, NASA researchers discovered that some meteorites that crashed into Earth contained loads of tiny nanometer-sized diamonds. Nanodiamonds are diamonds that are a few nanometers (billionths of a meter) in diameter. The researchers determined that 3 % of all the carbon found in meteorites came in the form of nanodiamonds. If meteorites are a reflection of the dust content in outer space, the astronomer’s calculations suggest that just a gram of dust and gas in a cosmic cloud could contain as many as 10,000 trillion nanodiamonds. These micro-gems are about 25,000 times smaller than a grain of sand, obviously much too small for use in jewelry. Astronomers believe that these tiny particles could provide valuable insights into how carbon-rich molecules, the basis of life on Earth, develop in the cosmos.
BIOMEDICAL USES FOR NANODIAMONDS
Will nanodiamonds play a role in creating longer-lasting and less-painful artificial joints? Having recently endured a nonelective MAKO total knee replacement surgery and the prolonged physical therapy required to regain as close to a normal range of motion and functionality as possible, I was excited to read a study by University of Alabama (Birmingham) researchers in the journal Acta Biomaterialia exploring the potential use of nanodiamonds in reducing wear on replacement joints made of metal alloys. The research suggests that nanodiamonds designed to toughen artificial joints might also help prevent the inflammation caused when hardworking metal joints shed metallic debris into the body.
This research is important because, according to the American Academy of Orthopedic Surgeons, more than 418,000 knee replacements and 328,000 hip replacements are performed in the United States each year; the numbers are expected to balloon as the nation’s population ages. Joint wear and tear generates debris that can cause pain, limit mobility and hasten artificial joint failure. Debris particles from metal surfaces are absorbed by scavenging immune cells called macrophages, which then secrete chemicals that cause swelling and pain. This inflammation “turns on” bone-eating cells near implants, and subsequent bone-loss increases the likelihood that joint implants will break loose and require a second surgery. Nanodiamond coatings may end the shedding of metal debris, but the constant grinding force within joints can cause even nanodiamonds to shed some particles. Thankfully, studies suggest that nanodiamonds shed less debris and smaller particles.
To help build global awareness, we would appreciate it if you would share this post with your friends and colleagues. Please choose one of the options below which includes email and print! Thank you.









I am so fascinated by your subjects, the research and work you put into the articles, and of course, the pictures. Your love of science is contagious – I especially love this glass in nature series – so educational. Thank you!
Diamonds, more and more fascinating! Shining even more….
Nanodiamonds and their medical use might save many people from post-surgical complications. Impressing.
Thank you very much, Audrey.
Bozena