ePostcard #137: Life in Glass Houses (Part 3)
Photo Credit: Courtesy of Hans Hillewaert and Wikimedia Commons. This amazing photograph of a tube-building marine worm (Lagis koreni) from the Oostendebank (Belgium) in the southern North Sea. The photo shows the sand grain-built tube and 24 mm-long trumpet worm that lives inside. Source: https://en.wikipedia.org/wiki/File:Lagis_koreni_(with_and_without_tube).jpg
SAND ARCHITECTS
We are not the only species that build homes and condominiums from sand. The Pectinariidae, also known as trumpet or ice cream cone worms, are a family of marine polychaete worms that build individual, cone-shaped tube housing using grains of sand and other small particles. Pectinariids construct their tube homes by reaching out with their tentacles and collecting individual grains of sand, which they glue together in precisely packed layers. This masonry style gives the tube a smooth appearance, almost like a colorful stained glass window or a tile mosaic. The tube protects and provides camouflage for the worm, housing the soft, fleshy body in a speckled coat of armor. Look closely at the photos above and below and you’ll see that the tube, which can be up to 2 inches long, is made out of thousands of tiny grains of sand, bits of snail shell and other particles. Thanks to the preservation of these silica-rich tube houses in the fossil record, paleontologists have identified the earliest known pectinariid fossils as Cretaceous in age.
Photo Credit: Courtesy of Malcolm Storey and National Museum of Wales. Ice cream cone worms, also known as trumpet worms, get their name from the carefully constructed, cone-shaped tube that they live in. Take a close look and you’ll see that it’s made from thousands of tiny grains of sand and bits of snail and other shells carefully glued together
Photo Credit: Courtesy of the Department of Ecology, State of Washington (https://ecology.wa.gov/Blog/Posts/July-2017/Eyes-Under-Puget-Sound;-Critter-of-the-Month). This photo compares the tubes constructed by two pectinariids, Cistenides granulata and Pectinaria californiensis, and shows the difference in preferred grain size between two species of ice cream cone worms.
HORSESHOE WORMS
Photo Credit: Courtesy of DiverKevin. A colony of Vancouver Phoronid (Phoronis vancourverensis) (https://www.diverkevin.com/NorthAmerica/Invertebrates-Eastern-Pacific/Invertebrates-Phoronida/i-9PJkxNs)
The Phylum Phoronida (the horseshoe worms) is one of the smallest and least familiar phyla of marine polychaetes. There are about 12 or so living species of phoronids in two genera, Phoronis and Phoronopsis, which are found mostly in shallow coastal waters. Each worm secretes an upright leathery or chitinous tube to support and protect their soft bodies, creating a “burrow” that it never leaves. The tubes may be anchored singly or in a tangled mass on rocks, shells, or pilings or buried in sand or mud. In sharp contrast to the precision-built tube cases of the trumpet worms, some phoronid species add a free-form jacket of agglutinated sand grains and bits of shell to to the tube in order to provide camouflage and protection from predators. On rocky substrates some species live in colonies and their tubes become twisted around each other for support to form a large and impenetrable mass.
Horseshoe worms filter-feed with a “crown” of tentacles known as a lophophore, which may appear as a simple ring in some species, folded into a horseshoe shape in others, as shown in the Vancouver Phoronid above (hence the common name “horseshoe worms”), or coiled. The horseshoe worm’s body remains hidden within their tube and all you can see are the many thin tentacles of the lophophore. The worm thrusts out the tentacles when feeding, but when disturbed it withdraws completely into its tube. The tentacles have numerous cilia on them, which the worm vibrates to generate a stream of mucous that entraps food particles that can then be drawn down the tentacles and into the mouth and digestive tract. As you can see below in Peter Grobe’s photos of the elegant horseshoe worm (Phoronis muelleri), the worm’s tube is extended beyond its quartz sand-encrusted outer casing and you can see the lophophore unfurled for foraging. In the second photo, the horseshoe worm is shown withdrawing back into its protective burrow.
Photo Credit: Both photos above are courtesy of Peter Grobe via Wikipedia/Flikr; phoronis-muelleri-photo-via-Peter-Grobe-flikr.
WINDMILL WORM
Members of the genus Praxillura (the “windmill” worms) are among the most common worms in soft sediment habitats on the seafloor, with a habitat range that extends from the intertidal zone to depths in excess of 1000 meters. These marine polychaetes are also called “bamboo” worms, because the worm’s anterior segments definitely bear a resemblance to bamboo shoots. Living in the sturdy tube that they construct in the seafloor, these worms are relatively large, 6 inches or more long, and ¼ in to ½ inch wide. The epidermis of the worm’s body is highly glandular and secretes a mucous material that hardens to form a very tough membrane-like material. When completed the tubes typically have fine to coarse sand, shell fragments, and small pebbles bound into their structure, which makes the tube quite strong.
The worms live in their tubes with the head facing the tube’s opening, which is ringed with the windmill-like spokes that make up the worm’s suspension-feeding apparatus. Extending out from the tube’s opening, the worm secretes a thin sheet of mucus that it attaches to all the spokes. With its net set, the worm retreats down into its tube and waits for food particles to get caught in the mucus. Periodically, the worm extends beyond the opening and eats the sheet of mucus and its attached particulate material.
Photo Credit: Both of the photographs above, courtesy of Dr. Ronald L. Shimek, are of a marine worm in the genus Praxillura. These unusual suspension-feeding marine worms were photographed in Hood Canal, Washington, at a depth of about 15 meters. Note that the end of the worm’s tube and its windmill-like arms are “armored” in a coating of sand or finer sediment and support a mucus sheet that captures food in the form of organic particulate material. The second photo shows a Praxillura maculata individual emerging to harvest the mucus and food particles.
SANDCASTLE WORMS
Photo Credit: Courtesy of Allison J. Gong and Bay Nature (baynature.org). This reef-like mound of sandcastle worm (Phragmatopoma californica) tubes houses hundreds of these marine worms and was photographed in the mid-intertidal zone in California’s Natural Bridges.
Many marine worms build handsome tubes of sand and shell, but the group known as the sandcastle worms have adapted to the ocean’s rough and tumble surf zone by constructing remarkable colonial “reef condominiums.” The California sandcastle worm (Phragmatopoma californica), one of the premiere tube-reef architects, is found in the intertidal surf zone from Sonoma County to northern Baja California (Mexico). This worm is dark brown in color with a crown of lavender tentacles and has a length of up to about 3 inches.
To survive in this dynamic environment, this resourceful worm fashions its rigid tube, a shelter that it never leaves, from grains of sand and tiny bits of scavenged shell glued together with an adhesive that the worm produces expressly for this purpose. For those of us that don’t dive, sandcastle worm tube-reefs are most visible at medium and low tide in intertidal habitats that provide the worms with some degree of shelter from the pounding surf, such as rock faces, overhanging ledges and concave shorelines. At low tide, the worm retreats into its tube and seals it shut with a shield-like operculum made of dark setae; at high tide, when the reef is submerged, its tentacles emerge to sift the water for food.
You might logically ask how these reef mounds get to be so big. As you can see in the photo above, the sandcastle worm’s reef-forming colony has a honeycomb-like outward appearance because each hole marks the entrance to an individual worm’s tube. Though surprisingly sturdy, sand tube construction isn’t as durable as the concrete we use in building a structure. So, for extra support, the worms build their individual abodes side-by-side, in tightly knit colonies that may reach many feet across. In essence, the entire reef structure is built tube-by-tube, worm after worm. Ultimately, these massive reef colonies are fortified over time by the gregarious settlement of sandcastle worm larvae, which require contact (chemical cues) with an existing colony in order to metamorphose into their adult form.
Fascinated by sandcastle worms, I began searching the scientific literature and discovered some remarkable research being conducted by Dr. Russel Stewart, Associate Professor of Bioengineering, and his colleagues at the University of Utah. Observations in Stewart’s laboratory confirmed that the construction process is meticulous, with the worm using its tentacles to select each grain of sand for its tube. Using a specialized organ on its head, the worm mason dabs a microscopic dot or two of its own “superglue” on the grain that it places, just so, on the existing tube structure. Holding each grain in place for around 25 seconds, the sandcastle worm may move the grain a bit to ensure that it is snuggly in place, just as we do when gluing two things together. By the time the glue on the grain is set, the next one has already been prepped for placement on the tube wall.
Photo Credit: Both courtesy of Fred Hayes and Dr. Russel Stewart at the University of Utah; [http://www.unews.utah.edu/p/?]. This sandcastle worm (Phragmatopoma californica) in Russell Stewart’s laboratory aquarium is making a tube out of sand (yellow) and beads of zirconium oxide (white).
SEM Photo Credit: Excerpted from a research article by R. J. Stewart, C. S. Wang, and H. Shao, Adv. Colloid Interface Sci. 167(1), 85 (2011).
Recognizing the potential medical applications of such a natural superglue, Stewart and his fellow researchers set about trying to understand how this glue works and how it might be synthesized. Since sandcastle worms seem happy to work with whatever material is available and so, in the laboratory, Stewart provided the worms with sand grain-sized glass beads which they proceeded to busy themselves with. The first photo below shows a worm tube started with natural sand and extended with the glass beads the researchers provided; the second photo is a scanning electron microscope image of the extraordinary care and accuracy with which the beads are glued together.
Dr. Stewart and his colleagues are a handful of researchers around the country who are developing adhesives that work in wet conditions, with worms, mussels, barnacles and other marine creatures as their model organisms. “Man-made adhesives are very impressive,” said Stewart. “You can glue airplanes together with them. But this animal has been gluing things together underwater for several hundred million years, which we still can’t do.” The goal of these researchers is not to duplicate natural adhesives that work well underwater, but to imitate them and make glues that are even better suited for humans. “We want to take elements of the structural adhesives that chemists have made and combine them with the unique elements that nature has used,” Dr. Stewart said.
FRESHWATER CADDISFLIES
Having worked with sandcastle worms since 2004, Dr. Stewart and his colleagues recently began studying another group of tube-building animals, caddisfly larvae. Caddisflies belong to the Phylum Arthropoda (insects, spiders, crustaceans and their relatives), and the Order Tricoptera, which includes distinctive families of moth-like insects with freshwater aquatic larvae and terrestrial adults. There are approximately 14,500 described caddisfly species in 45 families worldwide. Fly fishermen are especially familiar with these organisms, which inhabit the bottom of freshwater streams and rivers until the adult flies hatch. Artificial flies are tied to imitate the caddisfly adults, while larvae and pupae are collected and used as bait.
Caddisflies build their tubes in much the same way as the California sandcastle worm, but they use a very different glue — strands of silk that they attach to the bits of sand they collect, tying them all together. According to Dr. Stewart, “At some evolutionary point tens of millions of years ago the caddisflies were related to silkworms, so the fact that they spin silk is not too surprising. Except it’s a sticky, underwater silk.” Case-building caddisfly larvae use the silk to construct portable, tube-shaped shelters from a variety of materials (some cases are made exclusively of silk). The cases effectively protect the larvae from predators and from the abrasion caused by being tumbled about in the current. If disturbed, the larva can retreat into their case, which is constantly being repaired when damaged, or rebuilt as the larva grows. Those caddisfly species that do not build portable tubular cases, use the silk fiber they produce to make capture nets that they attach to the stream bed in order to filter food from the flowing water or use it to anchor themselves to the stream or river bottom to avoid being washed downstream while foraging. The key to the success and global distribution of caddisflies is attributed to their ability to produce this versatile silken thread, which is also why researchers are interested in the silk as a potential biomedical substance.
Caddisfly silk is made of large fibroin proteins, which play a key role in the larvae’s ability to make silk underwater (from research by Stewart and Wang, 2010). Caddisfly larva produce this silken adhesive from two glands located at the apex of their lower lip (labium). The silk fibers spin out from the caddisfly’s spinneret as flat ribbons, with a thin gluelike adhesive coating over the silken fiber core. Since the caddisfly’s silk is able to bond to a wide range of surfaces underwater, both organic and inorganic substrates, it has been the focus of medical research attempting to synthesize a silken bioadhesive (for humans) that sticks to wet tissues.
The life cycle of the caddisfly is as amazing as the protective cases they construct. As the caddisfly larva grows during the winter, it undergoes five molts, shedding the entire exoskeleton as a new one forms. As the larvae mature and elongate, they have to continuously add material to the case—think of it like adding rooms to your home for the rest of your life, or at least until you turn into an adult. If the caddisfly larva somehow loses its case, it’s got to start from scratch, and that’s quite the precarious situation for a soft bodied creature. The final larval molt coincides with the metamorphosis from larva to pupa, setting the stage for drastic tissue rearrangement and the mass migration of cells to produce the hairy wings and antennae of the adult. Stout mandibles grow solely to liberate the adult caddisfly from the silk-lined case, which would otherwise become a tomb.
Photo Credits: Courtesy of the Clean Water Education Partnership (CWEP) and Jan Hamersky. Case-building caddisfly larva (Trichoptera)
Photo Credit: Courtesy of Gerry Lemmo and the article Caddisflies: Underwater Architects by Declan McCabe in the Spring 2019 edition of Northern Woodlands Magazine. This adult sedge caddisfly (Pycnopsyche subfasciata) is a fall hatching species, its camouflage nearly perfect amid a tangled confusion of fallen leaves. Species in this genus are nicknamed the “great brown autumn sedges” by fly-fishers. At about three-quarters of an inch in length, they make a tempting meal for a hungry trout and fishermen often time their trips to coincide with a hatch.
MICROPLASTIC CHALLENGES
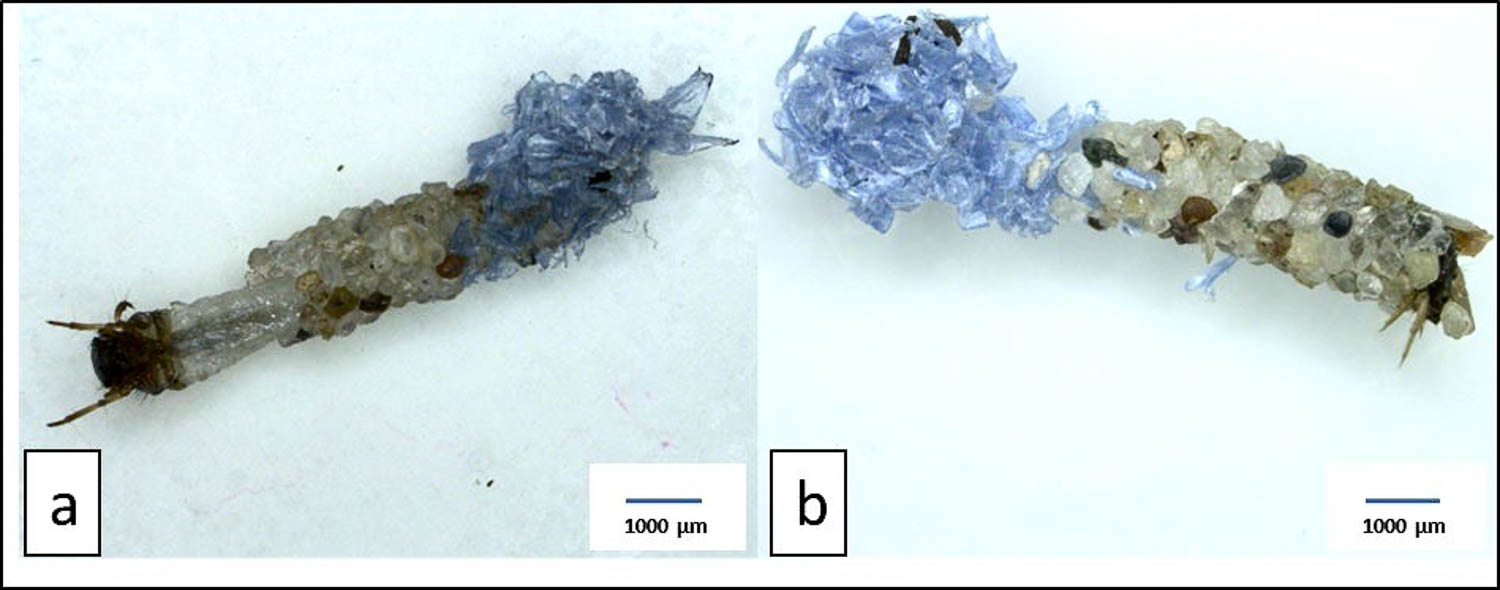
Microplastic pollution is now adding to the caddisfly’s environmental challenges in freshwater ecosystems. Microplastic particles—pieces of plastic under 5 millimeters long—have already impacted many of Earth’s environments, including the formerly pristine Arctic and deep-sea sediments. In a study published last year (cited below), researchers in Germany reported finding microplastic particles in the cases of caddisflies in the wild. Then, last month, they published the troubling results of lab experiments that found the more microplastic particles a caddis fly larva incorporates into its case, the weaker that structure becomes. This structural weakness might make caddisfly larvae more vulnerable to predation, sending ripple effects through river ecosystems. Researchers have been massively ramping up their work to understand how ingested microplastic particles might affect the physiology and behavior of animals, but relatively little has been done to determine how those particles might affect the structures that insects like caddis flies, bees, and termites build.
Photo Credit: Courtesy of researcher Tamara Al Najjar. Here you see two examples of caddisfly cases: (a) was collected from the wild, and (b) was built from sand by a larva in the lab The researchers used two kinds of plastic in this new experiment, polyvinyl chloride, which you know as PVC, and polyethylene terephthalate, or PET, a clear plastic used to make plastic bottles. In the lab, they chopped up the plastics into tiny pieces, which they mixed with sand in various concentrations. Then they let the caddis fly larvae do their thing.
Source: Ehlers, S.M., Al Najjar, T., Taupp, T. et al. PVC and PET microplastics in caddisfly (Lepidostoma basale) cases reduce case stability. Environ Sci Pollut Res 27, 22380–22389 (2020). https://doi.org/10.1007/s11356-020-08790-5
To help build global awareness, we would appreciate it if you would share this post with your friends and colleagues. Please choose one of the options below which includes email and print! Thank you.















Simply amazing! Always enjoy being blown away by you(r incredible writing) and the fascinating world we live in. I’ll be searching for caddisfly tubes while out fishing…
Fascinating! Again, nature finds incredible solutions.
Thank you, Audrey.
How do you find such fascinating creatures? There is so much we don’t know in this world.